A summary of results from SEQUOIA-HCM: A clinical study of aficamten for obstructive hypertrophic cardiomyopathy
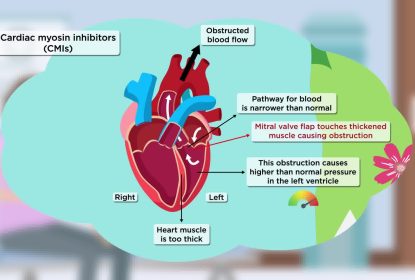
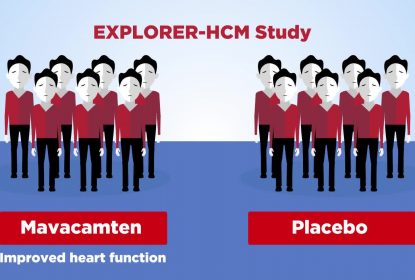
This video explains key findings from the SEQUOIA-HCM trial, which were published in the New England Journal of Medicine. SEQUOIA-HCM focuses on obstructive hypertrophic cardiomyopathy (or oHCM), a condition where the heart contracts too strongly and the heart muscle is too thick and stiff on the left side, leading to obstructed blood flow out of the heart. Aficamten is a cardiac myosin inhibitor that decreases how strongly the heart contracts, and is being tested in clinical trials as a treatment for oHCM. SEQUOIA-HCM involved 142 people with oHCM who took aficamten and 140 who took a placebo, plus their usual medicine(s). Researchers compared aficamten to placebo in terms of how well it works and how safe it is. After 6 months’ treatment, people who took aficamten had a greater improvement in exercise capacity compared to those who took a placebo. They also had improved quality of life, reduced symptoms, and an improvement in how well their heart worked. People had a similar number of side effects whether they took aficamten or a placebo. These results from SEQUOIA-HCM could mean that aficamten may become a new treatment to improve exercise capacity, symptoms, and quality of life among people with oHCM.
Original Article
Maron MS, Masri A, Nassif ME, et al. Aficamten for symptomatic obstructive hypertrophic cardiomyopathy. N Engl J Med. 2024;390(20):1849-1861. doi: 10.1056/NEJMoa2401424
Publication DOI: https://doi.org/10.1080/vjbm-2025-0003
Maron MS, Olivotto I, van Sinttruije M. A summary of results from SEQUOIA-HCM: a clinical study of aficamten for obstructive hypertrophic cardiomyopathy (2025). Video Journal of Biomedicine. 9(8). DOI: 10.1080/vjbm-2025-0003