Safety and efficacy darolutamide in Black/African-American patients in the ARAMIS study
In this video, we present outcomes of 52 Black/African-American patients who took part in the ARAMIS study.
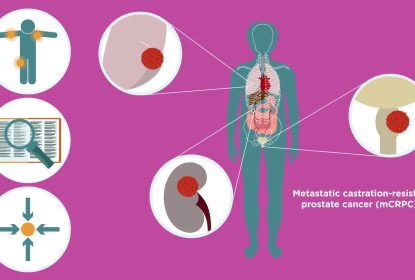
The ARAMIS study investigated the efficacy and safety of the androgen receptor inhibitor darolutamide in combination with androgen-deprivation therapy (ADT) in patients with nonmetastatic, castration-resistant prostate cancer (nmCRPC). The results showed that adding darolutamide to ADT prolonged the time that patients were free from their cancer spreading and helped patients live longer, compared to patients who only received ADT. You can find out more about the ARAMIS study here or by reading the ARAMIS plain language summary.
Prostate cells have a protein called an androgen receptor that responds to testosterone, which is a type of hormone known as an androgen. Inside the prostate cell, testosterone is converted to a slightly different hormone called dihydrotestosterone. Dihydrotestosterone attaches to the androgen receptor and sends a signal that causes the cell to grow. ADT, also known as hormone therapy, lowers testosterone/dihydrotestosterone levels in the body, leaving less available to attach to the androgen receptor and helping slow cancer cell growth.
Darolutamide attaches to androgen receptors, blocking testosterone/dihydrotestosterone signals and slowing tumor growth. nmCRPC is a stage of prostate cancer that no longer responds to ADT and has not spread to other body parts.
Black/African-American patients experience higher incidence and mortality rates of prostate cancer and are often underrepresented in clinical trials.
Original article:
Efficacy and safety of darolutamide in Black/African–American patients from the phase III ARAMIS study
Shore ND, Cruz F, Nordquist L, et al
Future Oncol. (2023) doi.org/10.2217/fon-2022-0943